Automate MedTech products efficiently – with scalable system solutions
With the highest quality standards as well as efficiency and scalability, Baumann automation systems deliver precisely what manufacturers in the MedTech sector require.
Our automation solutions create the foundation for safe, flexible and future-proof production. They are precisely tailored to the specific needs of the life-science industry.
Our expertise
With more than 40 years of automation experience, we provide end-to-end system solutions for the assembly, testing and packaging of MedTech products. We rely on our proven, modular automation platform ro|box MedTech, which is flexibly configurable, cleanroom-compatible and was developed specifically for use in GMP-regulated production environments. Our support during product design helps you unlock automation potential and sustainably reduce process costs.
Your benefits with our MedTech solutions:
GxP-compliant: Our systems are designed for use in GMP-regulated environments and meet all relevant quality and documentation requirements.
Maximum process reliability: With integrated testing and monitoring systems, we ensure the quality of every single product is completely assured and traceable.
Clean production conditions: By integrating laminar-flow systems, our solutions meet cleanroom requirements in accordance with ISO 5 and GMP Grade A, ensuring a particle-free environment for the highest quality standards.
ESD protection: Our automation systems can be equipped with ESD components to reliably safeguard sensitive electronics against electrostatic discharge.
Scalable ro|box MedTech platform: Easily adapts to production volumes and product requirements – from small batches to high-volume manufacturing.
Modular, standardised cells: A modular building-block system enables fast and efficient integration into existing production processes.
Innovative technologies: We combine robotics, sensor technology, and cutting-edge digital solutions to create intelligent systems in compliance with EU GMP Annex 11 and FDA 21 CFR Part 11, optimally aligned with networked manufacturing processes.
Focus on sustainability: Energy-efficient drive technology, durable components, and optimized processes reduce resource consumption and operating costs.
Global support: Our international service network provides reliable support throughout your system’s entire lifecycle.

Relevant assembly and testing technologies for medical technology automation
We offer a wide range of established processes, including:
- Welding (e.g. laser, ultrasonic, resistance)
- Soldering
- Bonding and dispensing
- Heat staking
- Press-fitting
- Screwdriving
- Surface treatment (e.g. plasma, CO₂)
- Part feeding (e.g. tray, bulk material)
- Electrical testing (e.g. ICT, flash, EOL)
- Optical inspection
- Leak testing
- Labelling, tagging and laser marking
- Packaging and palletising
- and many more ...
Application examples for automation in medical technology

Pacemakers & neurostimulators
Micro-assembly with inline functional testing, laser welding, helium leak testing, and UDI marking.

Injection systems & insulin pens
Automated multi-component assembly with sealing, mechanical components, dosage control, and safety lock.
Sensors & wearables
SMT placement, micro-encapsulation, laser marking and calibration processes.

Cleanroom-compatible disposables
Feeding, assembly and packaging under cleanroom conditions with full traceability.
ro|box medtech automation platform

What sets ro|box medtech apart?
- Stainless-steel enclosures (V4A): Robust, corrosion-resistant, easy to clean and resistant to cleaning/disinfectant agents.
- Polished, sealed surfaces: Reduce build-up and prevent microcracks or porosity that could harbour germs.
- Avoidance of dead spaces/corners: No hard-to-reach or poorly visible areas where particles or liquids could accumulate, or where parts or components could remain undetected during line clearance.
- Hygienic design to EHEDG/GMP guidelines
- Rounded edges and corners: Facilitate cleaning and prevent dirt accumulation.
- ESD protection components (optional): Prevent electrostatic discharge for sensitive electronic components.
- Laminar-flow systems (optional): Particle-controlled environment, suitable for cleanroom classes up to ISO 5 / GMP Grade A.
- Validation according to GMP/FDA guidelines: Technical design compliant with regulatory requirements (e.g., ISO 13485, EU GMP, FDA 21 CFR, and ISPE GAMP 5).
- Frameless glass doors on all sides: Simplified cleaning and visual inspection during operation.
- Use of EHEDG-certified equipment: Including handles, control cabinet locks, screws, nuts, etc.
- Stainless-steel base plate
- Use of clean to highly aseptic robot arms
- Wipe-down-capable/manual cleaning ready: Hygienic design prepared for common disinfectants and cleaning agents.
- Chemical-resistant materials: Suitable for alcohol-, acid-, and alkali-based cleaning.
- Chemical-resistant seals: (EPDM, FKM, PTFE).
-
Integrated audit trail
High flexibility & scalability
- Production can be quickly adapted to new volumes, variants or product life cycles.
Modules can be added, removed, or replaced under change control without the need to purchase an entirely new system.
Faster time-to-market
- Preconfigured platform modules shorten engineering and commissioning times.
Cost efficiency over the product life cycle
- Lower investment costs through reuse of base modules.
Maintenance and upgrades are easier and more cost-effective thanks to standard modules – with reduced risk.
Validated and proven modules reduce the likelihood of technical issues.
Simplified process validation.
Future-proofing via modular expandability – standardisation and quality.
Unified modules ensure consistent quality and a harmonised HMI concept.
Reduced training effort for personnel.
Spare parts management and service are simplified.

GMP-compliant engineering for the medical device industry
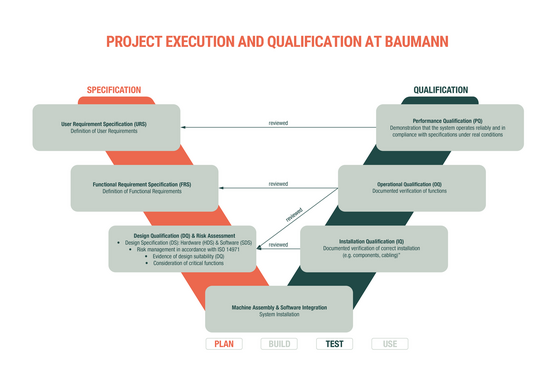
We guarantee GMP-compliant design and the consistent application of Good Engineering Practice at every stage of the project.
From the outset through to project completion, we support you in all qualification steps and provide the required documentation in a customer-specific format.
Preconfigured platform modules shorten both engineering and commissioning times.




